
We know the electron configuration and that all the orbital sets are full (all electrons paired) through 4d. It illustrates the rules of filling.Īnswer: THREE. Its an Electron Energy Diagram (Aufbau Filling Order).īelow is an animated gif showing the proper filling for the 2nd row of the periodic table. Here is a nice pdf for you to use to practice dropping in electrons (arrows) in the right order. Pauli Exclusion Principle - no two electrons can have the same set of four quantum numbers - these means no more that 2 electrons per orbital (blank) and when there are two, they have opposite spins ⥮.Note: electrons with matching spin states are said to have parallel spin states Hund's Rule - on any degenerate levels (same energies), always fill singly with up arrows ↿ (+½) before you then pair with the down arrows ⇂ (–½).Aufbau Principle - always take the lowest possible energy level and fill it before going up to the next level.The orbitals themselves are shown on an energy diagram as blanks and we will put in up arrows ↿ and down arrows ⇂ to represent the spin quantum numbers +½ and –½.
#Antimony orbital diagram how to
Once you memorize and can use the periodic table to help you get the correct order of orbitals, you then need to know how to fill those orbitals. Next thing to remember is how many electrons go into each orbital set and also the ordering of spin within a set. Oh my, how are you going to remember all that? Well, the best way to memorize the aufbau filling order is to use a periodic table and know how the orbitals "fit" the table. Here is the order of filling for all the orbitals in the atom.ġs 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p
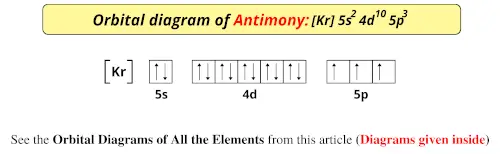
We will completely fill a lower level of energy before we advance to the next higher level. Pour water in a bucket and it fills from the bottom up - same idea. The aufbau principle tells us to "build up" from the bottom of the energy well to the top. NO duplicates! It's like a serial number for electrons, except we use n, ℓ, m ℓ, and m s. The Pauli exclusion principle says that all electrons in an atom have to have a unique set of quantum numbers.


 0 kommentar(er)
0 kommentar(er)
